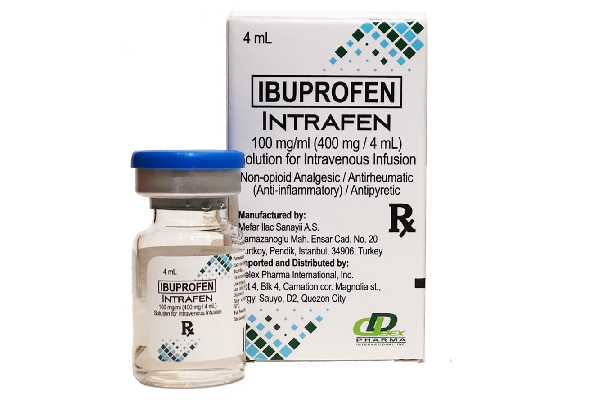
A Classic Ibuprofen Reinvented
The first and only Ibuprofen brand in I.V. preparation available in the market.
ABOUT INTRAFEN

NSAIDs in Post-operative Pain
Acute pain is a significant problem for in-patients and can occur secondary to acute illness or disease processes, trauma, or operative procedures. In the postoperative period, 80% of individuals suffer from post-operative pain with almost all describing it as moderate to severe.
Opioid analgesics are the mainstay in the management of post-operative and acute pain in the inpatient setting. However, its use is often limited by adverse effects including respiratory depression, sedation, allergic reactions, and gastrointestinal events. That is why adjunct agent such as NSAIDs is used in combination with an opioid as it helps to lower the side effects of both agents by reducing the dose required.
Ibuprofen as a Safer NSAID
Ibuprofen is a non-selective COX inhibitor. Studies proved that the Adverse Event (AE) profile of Ibuprofen is better since COX-1: COX-2 ratio is lower4. It shows a lower side effect profile on gastrointestinal and cardiovascular systems due to its balanced COX1 and COX2 inhibition5
IV Ibuprofen (INTRAFEN) in the Philippines
Reliable clinical studies have proven the potential or efficacy of Ibuprofen in addressing post-operative pain and fever reduction. Hence, Delex Pharma acquired the exclusive distributorship and marketing of INTRAFEN in 2017. This deed supports the company’s vision in serving the Filipinos with quality critical care medicines. Delex is proud of making INTRAFEN the first and only IV Ibuprofen in the Philippines. It is indicated for adults and children in the management of 1) mild to moderate pain 2) moderate to severe pain as an adjunct to opioid analgesics and 3) reduction of fever. It may be used perioperative except in patients undergoing CABG surgery7.
Intrafen is a solution for intravenous infusion available in 100 mg/mL (4 mL and 8mL) vial. Remarkably, thirty-five (35) private hospitals in the country trust the potency of the product. Delex expands its availability in selected Mercury Drug branches to address its rising demand.
Intrafen is manufactured by GEN ILAC of Turkey. The cGMP-compliant facility meets the international regulatory guidelines of European Union Good Manufacturing Practices (EU GMP) and the US FDA.
Sources: 1.Rathmell JP, Wu CL, Sinatra RS, et al. Acute post-surgical pain management: a critical appraisal of current practice, 2005 Dec 2–4. Reg Anesth Pain Med. 2006;31:1–42. 2. P Brandon Bookstaver, et al. Intravenous ibuprofen: the first injectable product for the treatment of pain and fever, Journal of Pain Research 2010:3 67–79. 3. http:// americanpainsociety.org/about-us/press-room/american-pain-society-publishes-clinical-practice-guideline-for-postsurgical-pain-management. 4. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease, 8th ed., Copyright© 2006 Saunders, An Imprint of Elsevier. 6. Bushra R, Aslam N. An Overview of Clinical Pharmacology of Ibuprofen. Oman Medical Journal 2010, 25 (3): 155-161. 7. Scott JL. Intravenous Ibuprofen In Adults For Pain and Fever. Drugs 2012; 72 (8): 1099-1109