SAFETY
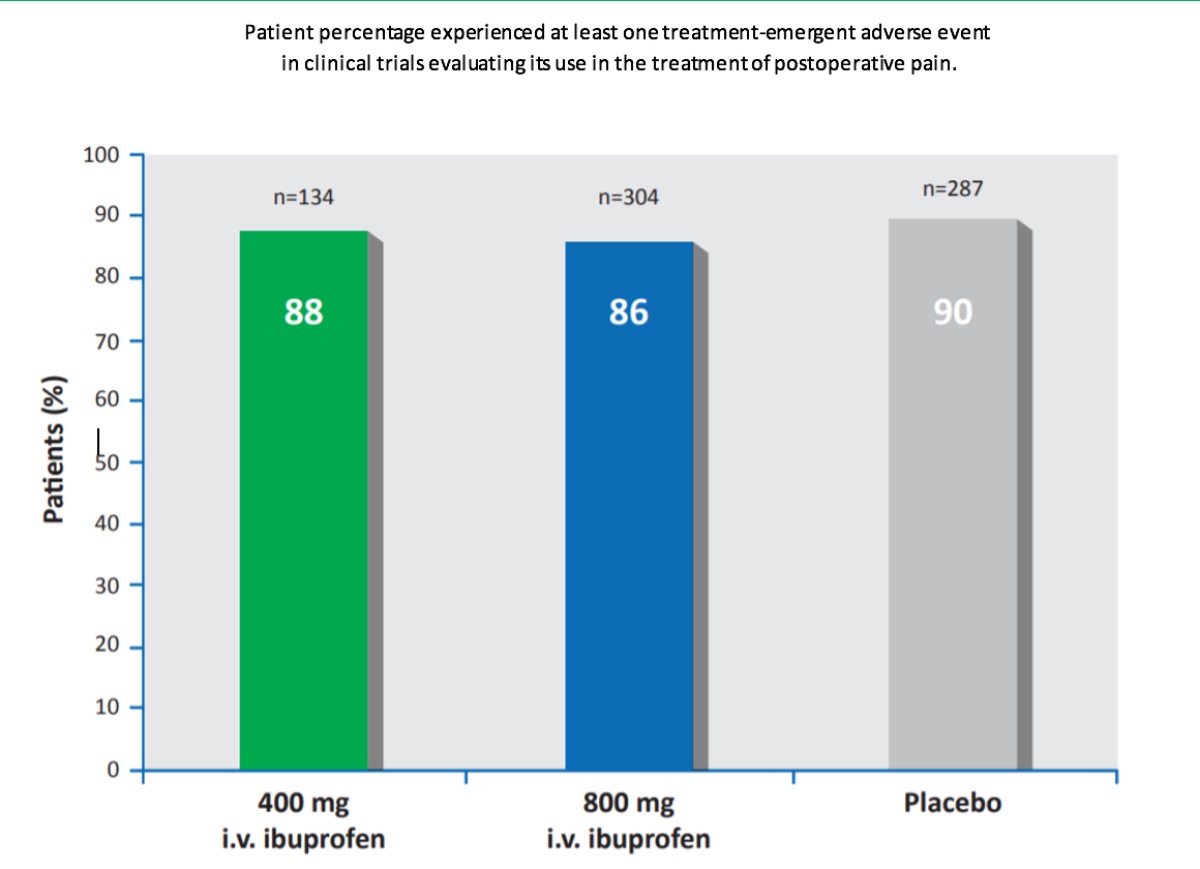
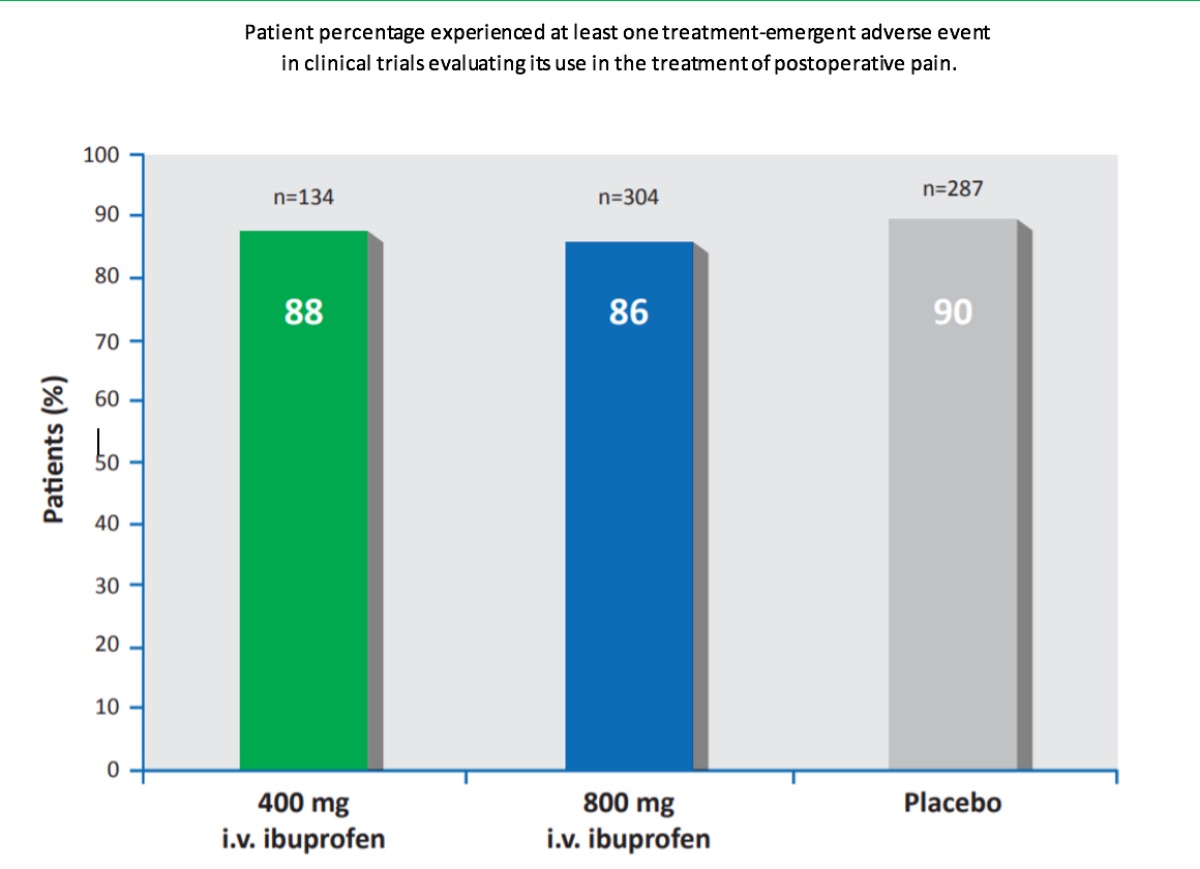
Intravenous ibuprofen was commonly well tolerated by patients in the multiple clinical trials evaluating the efficacy of intravenous ibuprofen for pain and fever treatment. In pooled analyses, the majority of patients receiving intravenous ibuprofen or placebo experienced in clinical trials evaluating its use in the treatment of postoperative pain (88% [400mg ibuprofen/dose; n=134] and 86% [800mg ibuprofen/ dose; n = 304] of patients in the ibuprofen groups and 90% of patients in the placebo group [n=287]) or fever (74–87%[100– 400 mg ibuprofen/dose; n=91] and 89% [placebo; n=28].
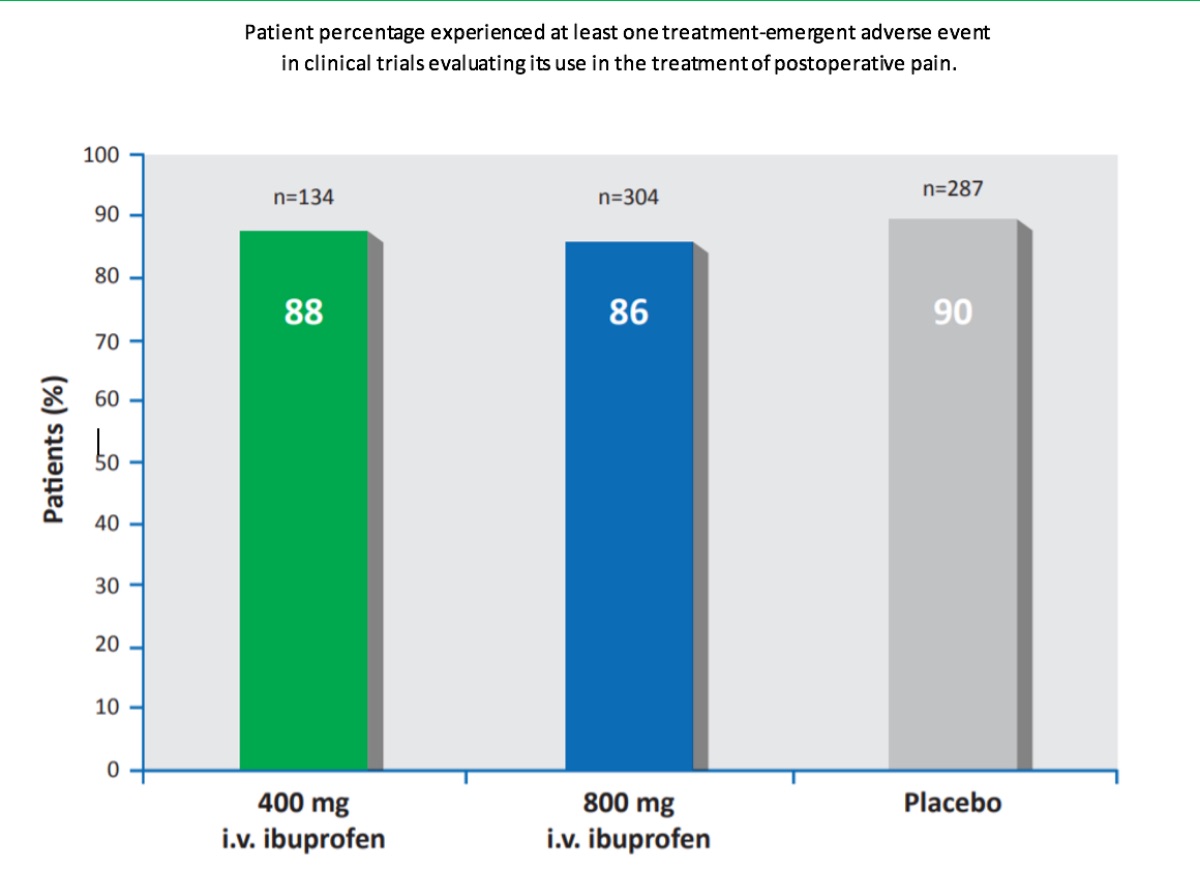
Intravenous ibuprofen was commonly well tolerated by patients in the multiple clinical trials evaluating the efficacy of intravenous ibuprofen for pain and fever treatment. In pooled analyses, the majority of patients receiving intravenous ibuprofen or placebo experienced in clinical trials evaluating its use in the treatment of postoperative pain (88% [400mg ibuprofen/dose; n=134] and 86% [800mg ibuprofen/ dose; n = 304] of patients in the ibuprofen groups and 90% of patients in the placebo group [n=287]) or fever (74–87%[100– 400 mg ibuprofen/dose; n=91] and 89% [placebo; n=28].
As with all NSAIDs, ibuprofen may increase the risk of serious cardiovascular thrombotic events, myocardial infraction or stroke, some of which may be fatal. The risk of such events may increase with the duration of NSAID use. Controlled clinical trials of COX-2 selective NSAIDs for the treatment of pain in the first 10–14 days following CABG surgery demonstrated an increased incidence of myocardial infarction and stroke.
NSAIDs, including ibuprofen, may cause serious and potentially fatal gastrointestinal adverse events, including inflammation, bleeding, ulceration and perforation of the stomach, small or large intestine. These events occur in approximately 1% of patients treated with NSAIDs for 3–6 months and 2–4% of patients treated for longer periods. In clinical trials, elevations of ALT or AST levels of approximately ≥3x the upper limit of normal were reported in approximately 1% of patients receiving NSAIDs. There have also been rare and potentially fatal cases of severe hepatic reactions, including jaundice, fulminant hepatitis, liver necrosis and hepatic failure. Treatment with NSAIDs, including ibuprofen, may lead to the onset of new hypertension or worsening of pre-existing hypertension, which in turn, may contribute to the increased incidence of cardiovascular events. Some patients experience fluid retention and oedema during NSAID treatment; as with other NSAIDs, ibuprofen should be used with caution in patients with fluid retention or heart failure.
Ibuprofen, like other NSAIDs, may cause serious and potentially fatal skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome, and toxic epidermal necrolysis; treatment should be discontinued if rash or other signs of local skin reaction occur.
It is this enzymatic inhibition of COX-1 and COX-2 that also leads to the hematologic, renal, and gastrointestinal adverse events associated with NSAIDs. Other side effects are idiosyncratic and less directly related to COX inhibition. COX-1 is responsible for the production of thromboxane A2, which is expressed in mature platelets. Thromboxane A2 acts as a platelet aggregator and enhances the effect of other platelet aggregators, including thrombin. While aspirin acts as an irreversible inhibitor of COX-1 and thromboxane A2 production in a dose-dependent manner, the activity of NSAIDs on platelets is temporary and reversible. It is incomplete inhibition of platelet aggregation that is believed to lead to the increased incidence of thrombotic adverse events with NSAIDs, including ibuprofen.
Within the kidney, COX enzymes synthesize prostaglandins in the medulla and cortex. They play a key role in blood pressure regulation and maintenance of renal blood flow, especially in patients with long-standing chronic kidney disease or who are volume depleted. These effects are what often lead to the hypertension seen with NSAID therapy. The vasodilatory effects of prostaglandins and COX enzymes maintain renal blood flow and glomerular filtration, and inhibition of their production leads to decreased perfusion and a decline in renal function. Synthesis of PGE2 is cytoprotective within the gastrointestinal (GI) tract and is protective against peptic ulcers, even without a reduction in acid secretion. Inhibition of COX-1 is thought to be responsible for the increased incidence of peptic ulcers and GI bleeding seen with NSAID therapy. Ibuprofen is non-selective, and its inhibition of COX-1 and COX-2 means that it is prone to GI side effects.
Bronchospasm can also be observed with NSAID therapy and is more prevalent in patients with a history of asthma or
reactive airway disease. Inhibition of the COX enzymes by NSAIDs shifts metabolism of arachidonic acid from PGE2
and PGI2, which relax bronchial smooth muscle. Arachidonic acid instead is synthesized into leukotrienes that constrict
airway smooth muscle.
Southworth and colleagues determined that 91% (368/406) of patients experienced treatment-emergent adverse
events when comparing morphine PCA combined with 400 mg or 800 mg of intravenous ibuprofen or placebo. Nausea,
vomiting, and constipation occurred most frequently in all study arms including placebo with fewer side effects
observed with ibuprofen. There was significantly less nausea with 400 mg ibuprofen relative to placebo. This apparent
reduction in gastrointestinal side effects is likely related to a reduction in opioid use and a corresponding reduction in
their adverse effects. Pyrexia was also significantly reduced in the 400 mg and 800 mg ibuprofen arms as compared to
placebo. In the 800 mg ibuprofen arm, a statistical reduction was found in the incidence of dizziness as compared to
placebo. Laboratory values including renal function and vital sign measurements were similar throughout the study in
all three groups. The incidence of bleeding and bruising did not differ significantly between the groups. Overall, the total
blood units transfused during the study were similar amongst all three arms. Of the 406 patients treated in this study,
there were no deaths reported. Discontinuation of the study drug occurred on day 5 of 14 for all 406 patients. Most of
these patients were able to tolerate oral pain medication on day 5, and therefore the intravenous therapy was
discontinued.
In 319 female patients undergoing elective abdominal hysterectomy, randomized to receive 800 mg intravenous ibuprofen every 6 hours or placebo, there were no differences in treatment-emergent adverse events. Clinical laboratory assessments including renal and hepatic function were similar between the two groups and the most common side effects were similar to those associated with oral ibuprofen use. Serious adverse effects were determined to be related to the hysterectomy procedure itself, and not study drug. Similar results were found in the 185 patients undergoing orthopaedic elective knee or hip surgery, with the exception of a difference in gastrointestinal side effects. There were significantly more patients who experienced emesis in the ibuprofen group as compared to placebo, however significantly more patients in the placebo group reported dyspepsia.
A study of hospitalized burn patients found no significant differences between the group treated with intravenous ibuprofen 800 mg and placebo. No significant differences in the incidences leucocytosis and anemia were found. Five subjects experienced 6 serious adverse events in the ibuprofen group and 3 subjects experienced 6 adverse events in the placebo group. There was no difference in the clinical laboratory assessments (serum creatinine levels, hemoglobin, platelet counts, prothrombin time, or activated partial thromboplastin time) between the two groups. The frequency of blood transfusions was similar in both groups. Findings from this study were similar to previous studies and demonstrated a relatively favorable side effect profile.